COSMIC ORIGINS OF EARTH MATERIALS
Earth science is interdisciplinary - it relies on a basic knowledge of other sciences such as chemistry, physics, astronomy, and even biology. To understand earth science, it helps to understand the origins and basic theories behind what earth materials (elements, compounds, minerals, rocks, etc.) are composed of and where they originally came from.
BASIC PHYSICS
The science of physics concentrates on the interactions between matter and energy. All that exists in totality is defined as the Universe. The Universe, to a physicist, is composed either of matter or energy.
Matter is defined as material that has substance, with a measurable property known as mass. ("Mass" is proportional to the size or volume of matter, and is related to the property known as "weight," which is the mass under the influence of gravity. Mass is constant, while weight is influenced by gravity; for example, a 200 pound human would weigh over a thousand pounds on Jupiter because of its larger mass and heavier gravity.) There are 4 basic states of matter: solid, liquid, gas, and plasma. Plasma is extremely hot, ionized gas, as found in stars. There also exists in the Universe an enormous amount of apparently empty space containing no detectable matter or energy.
Energy is defined in physics as "the capacity of a physical system to do work." Energy, unlike matter, contains no mass, and is measured usually by its effect on matter. "Work" in the physicist's definition means a measurable change in the "system" or local environment that is under observation.
Physicists have long sought to define the mathematical relationships between different parts of the natural universe. Perhaps the most famous equation in physics is Albert Einstein's equation from his Special Theory of Relativity, which summarized the mathematical relationship between matter and energy:
To understand Einstein's equation in a qualitative (rather than quantitative) sense, it means simply that a tiny amount of mass (matter) can be converted into a tremendous amount of energy. The speed of light (C) is a stupendously large number; "squared" means to multiply this huge number by itself; the result, "C-squared" is such a large number that even when multiplied by a microscopic amount of mass, the resultant "E" (energy) will be a huge number. This is the explanation for nuclear energy, and explains what causes stars (including our sun) to shine.
THE BIG BANG & COSMIC ORIGINS
We will now borrow from the science of astronomy, the study of outer space. Astronomy is one of our oldest sciences, dating back some 4,000 years in human history. (Ironically, geology and earth science have only been around for 200 years.) Nowadays, many aspects of physics have been incorporated into astronomy, as the interdisciplinary field known as astrophysics. From astronomy, we will now look at the current theory of the origin of our Universe, known as The Big Bang.
Many years ago, astronomer Edwin Hubble made the startling discovery that all objects in space were moving away (receding) from the Earth, and that the more distant the objects, the more rapid the rate of recession. The explanation for his observations is consistent with an ancient cosmic explosion, later termed The Big Bang, whereby fragments closest to the explosion were more energetic and thus moving faster than fragments more distant from the explosion. To help you understand this concept, try this thought experiment:
Imagine that you could safely film an explosion, and that you could view this film in slow motion, played backwards. What would you see? You would see the exploded fragments slowly converging together towards a central, intact object. Now apply this conclusion to Hubble's observations. Would you not also conclude that the Universe had a point of origin in time before the explosion? The answer is yes.
You may wonder how Hubble was able to tell how distant stars and other objects could be receding. To understand this, you need to become familiar with a principle of physics known as the Doppler Effect. I will use sound waves as an example, and extrapolate this principle to light waves, which is what Hubble did.
All of us should be familiar with this experience: You are standing on a street corner, observing an ambulance racing by, with its siren blaring. How does the character of the siren's sound change from when the ambulance approaches, verses when it is receding from you? The pitch of the siren shifts and becomes higher when the ambulance approaches, and shifts to a lower pitch when the ambulance recedes.
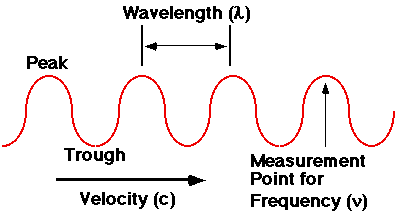
Most forms of energy, such as sound, and light, may be represented graphically as waves, which are mathematical forms that repeat their shape and pattern, as shown in this diagram:

The wavelength of a lower pitched sound is longer (wider), while the wavelength of a higher pitched sound is shorter (narrower). So, the pattern you should detect using the ambulance example is this:
"An approaching object shows a shift towards shorter wavelength sound; a receding object shows a shift towards longer wavelength sound."
Now, let's make the connection with Hubble's observations. Light waves are but a small part of the electromagnetic spectrum of energy that exists in the Universe. Have you ever noticed that all rainbows display the red band on the outer edge, while the blue band is on the inner edge?

Red light has a longer wavelength than blue light. So, if we substitute light waves in place of sound waves, the above pattern should be restated as:
"An approaching object shows a shift towards shorter wavelengths of light (blue-shifted), while a receding object shows a shift towards longer wavelength (red-shifted)."
Hubble's observations (verified by other scientists) have consistently shown a red shift of light is displayed by all observed objects in outer space. The red shift is most pronounced when viewing the most distant objects at the edge of the observable Universe. So, at some great distance from the Earth, we are not only looking back through a great distance, but also back in time.
In Space, Great Distances and Time Merge Together
Physicists tell us that the fastest that an object can move is at the speed of light, which is 186,282 miles per second. At this speed, a beam of laser light can reach from the Earth to the Moon's surface in just over 1 second. Stars and other celestial objects are so distant from the Earth that earthbound measurements such as the mile are too small to be useful. The distance traveled by a beam of light in one earth year is over 6,000,000,000 (6 trillion) miles, and this unit is called the light year. The distance from the Sun to the nearest star, Alpha Centauri, is about 4 light years. The Sun and planets in our solar system are located on the outer edge of the Milky Way Galaxy, which contains an estimated 100,000,000,000 (one hundred billion) stars. The Milky Way is shaped like a flat, elliptical disk, that is 30,000 light years across.
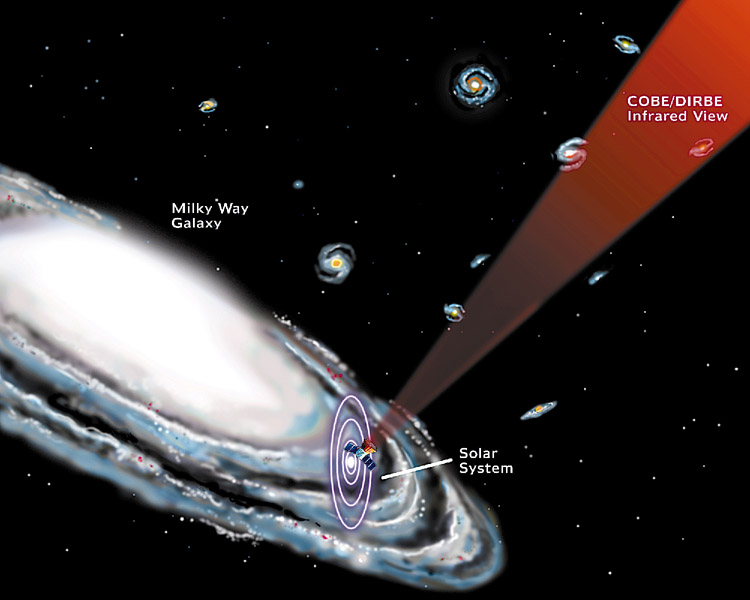
The distance to the next nearest galaxy, the Andromeda Galaxy, is nearly 2,000,000 (two million) light years away. When you view distant objects in the sky, you are looking back in time; the light that you see from Andromeda left that galaxy 2 million years ago. Astronomers now tell us that the Universe began about 13 billion (13,000,000,000) years ago, because that is the most distant part of the observable universe, after the cataclysmic explosion (The Big Bang) of what has been called the Cosmic Egg. All of the matter and energy of the Universe was created in that moment. However, it is believed that it took some time for the Universe to evolve into its present form. For the first 100,000 years or so, the Universe was much hotter than it is now, and contained much higher concentrations of mass and energy in a smaller space. Afterwards, stars and galaxies began to form, and the Universe has been expanding ever since, increasing the distance between every object in the Universe.
Origins of Stars
Stars are basically hot, luminous cosmic furnaces that fuse hydrogen into helium; in the process, a tiny amount of mass is converted into energy, which maintains the high temperature necessary for fusion to continue (over 2,000,000 degrees Celsius). Stars are classified according to their size and the amount of light they give off (luminosity). The Sun is characterized as a small to medium-sized yellow star, with an expected lifetime of 10 billion years, meaning the time span before the Sun runs out of hydrogen. Other stars, such as the blue or white super giants, are much larger, hotter, and brighter than the Sun, but have much shorter life spans (a few hundred thousand years). Still other stars are smaller than the sun, burn more dimly and have even longer life spans. Stars cooler than the sun glow orange or red. The most unusual stars form from the explosion of very large, hot stars, where all of the heavy elements are crushed into a 10-mile diameter sphere of neutrons, called a neutron star. A teaspoon of neutron star material would weigh more than the entire Earth. Some neutron stars emit x-rays, acting like a lighthouse beacon; astronomers called them pulsars. Some large stars collapse so completely that their overwhelming gravity prevents light from escaping; these are known as black holes. A supermassive black hole is thought to be at the center of our Milky Way galaxy.
The Sun is thought to have formed from a cloud of cosmic dust and hydrogen gas (called a nebula, created by a supernova, the explosion of a large star when it ran out of hydrogen to fuse) about 5 billion years ago. One of the most famous deep space photographs taken by the orbiting Hubble Telescope is the Eagle Nebula (M16), located nearly 7000 light years from Earth, stretching 4 to 5 light years across, which have been dubbed:

From Nebula to Solar System Formation
If another nearby supernova occurs, the violent explosive force compresses the diffuse gas of the existing nebula, increasing gas density and causing it gather into a common center through a process called accretion. As the dust and gas compressed, it began to spin, creating heat; when the center of this cloud reached a temperature of 2 million degrees Celcius, nuclear fusion reactions began, and the Sun was born. The Sun continued to accrete leftover matter; however, certain concentrations of the leftovers formed the planets, which established different orbits around the Sun. Other solar system leftovers included asteroids (flying mountains), meteorites (so-called shooting stars), and comets (cosmic snowballs), mostly concentrated in a vast asteroid belt between the orbits of Mars and Jupiter. Comets and ice-bearing meteorites and asteroids are now thought to have contributed a significant amount of the Earth's water early in its history.
The inner planets, closest to the Sun include Mercury, Venus, Earth, and Mars. They are characterized as rocky, or terrestrial planets. Much of their original hydrogen had been "blown" away by the Sun's solar wind early in its history, over 4.5 billion years ago. Most of Earth's hydrogen is found in compounds such as water and methane, as hydrogen is too light to be held in abundance by Earth's gravity. (Pluto, the farthest planet from the Sun, seems to resemble the terrestrial planets, and may have been a captured asteroid or moon.)
The outer planets, including Jupiter, Saturn, Uranus, and Neptune, are called the gas giants, because they are much larger than the terrestrial planets, having retained much of their original hydrogen by being far from the Sun. Jupiter is more than 1,000 times larger than the Earth, and some astronomers think Jupiter could have been a companion star to the Sun had it been about 10 times larger. It has a permanent "hurricane" known as the Great Red Spot that is larger than the entire Earth, and has existed for over 300 years. Saturn, famous for its rings (made up of ice and dust), is smaller than Jupiter, and has the lowest density of all the planets - less than that of water. (If there were a swimming pool large enough, Saturn would float.) Contemporary data indicates that the outer planets Uranus and Neptune are "ice giants" having an abundance of water, methane, and ammonia ice under a thick atmosphere of hydrogen and helium. Uranus is known for its unusual rotational axis, which is almost horizontal (it spins like a baker's rolling pin). Neptune is known for its deep blue color due to the presence of frozen methane. Pluto was considered the 9th planet from the Sun (not discovered until 1930), but recent advances in astronomy revealed the true size of Pluto to be only 1/6 the size of Earth; smaller than Earth's Moon. Based upon this evidence, in 2006, Pluto was demoted from a planet to a planetoid. So, as of 2006, there are only 8 planets in the solar system.
Some of the planets have satellites or moons in orbit around them. Mercury and Venus have no moons, but Earth, Mars, Jupiter, Saturn, Uranus, Neptune, and Pluto all have at least one moon. In terms of relative size between planet and satellite, Earth's moon is the largest.
The current theory of the formation of the Moon is
based upon extensive geochemical analysis between earth rocks and lunar
rock samples brought back from the Apollo space missions. Moon rock
is nearly as old as the Earth's, but lacks any evidence of an iron core.
It is believed that very early in the history of the solar system, when
there was a greater abundance of meteorites and asteroids, a very large
body (a Mars-sized planet called Theia) struck the Earth and was
destroyed; the energy of the collison caused the early Earth to become
molten. The ejected debris from the explosion went into orbit and formed
a ring around Earth, which re-accreted to form the Moon thousands of years
later. The Moon was originally much closer to the Earth than it is
today; it would have appeared 15 times larger in the sky, had any people
been around to observe it over 4 billion years ago.
CHEMISTRY AND COSMIC ORIGIN OF THE ELEMENTS
An element is a fundamental substance which cannot be further subdivided without destroying its identity. An atom is a particle of an element. All elements (over 109 are known) originated from stars long ago. The theoretical model of the atom, developed about 200 years ago, is fundamental to understanding chemistry. Atoms are depicted as microscopic spheres, composed of 3 types of subatomic particles, known as protons, electrons, and neutrons. Protons and neutrons reside in the nucleus at the center of the atom; they have about the same mass, but protons carry a positive (+) electrical charge, while neutrons are electrically neutral, and have no charge. Electrons are much smaller subatomic particles, having only about 1/1800 the mass of protons; being smaller and lighter, they are electrically attracted to the protons in orbits, moving at great speeds (depicted as an "electron cloud"). The size of a particular atom is defined by the orbit of its outermost electrons. Here is a diagram of the element Lithium, which has 3 protons (+), 4 neutrons (N), and 3 electrons ( - ):
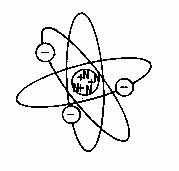
Simplified atomic model of the element lithium
The identity of an element is defined by the number
of protons, or atomic number. The atomic weight of
an element is the collective number of protons and neutrons. (Each
proton and neutron is arbitrarily given the value of 1 atomic mass unit.)
Common
hydrogen has an atomic number of 1 and an atomic weight of 1; it has no
neutrons. There are naturally occurring variations of elements that
have different number of neutrons, called isotopes.
Two isotopes of hydrogen are deuterium, which has 1 neutron, and
tritium,
which has 2 neutrons.
|
H 1 common hydrogen |
H 1 deuterium |
H 1 tritium |
The science of chemistry focuses on interactions between elements and/or compounds, caused by the transfer of electrons (tiny, negatively charged subatomic particles orbiting the nucleus of atoms) between different substances and the resultant changes in energy and composition. The science of geochemistry and cosmochemistry, respectively, deals with the relative abundances of each of the elements in the Earth, and in the cosmos. The matter of the Universe is believed to be about 70% hydrogen, the simplest of the elements.
Isotopes: Different Forms of the Same Element
Isotopes called radioactive isotopes are unstable; the nucleus undergoes a spontaneous disintegration (radioactive decay) at a fixed rate, which releases energy (radiation) and subatomic particles, leaving a smaller, lighter element as a by-product. Carbon-14 is a radioactive isotope which decays into common Carbon-12. Since they are both forms of carbon, each form has 6 protons, but Carbon-14 has 8 neutrons while Carbon-12 has 6 neutrons. Not all isotopes are radioactive; some are known as stable isotopes. Oxygen-18 is a stable isotope of common Oxygen-16. The existence of radioactive isotopes makes it possible to use it as an age measurement tool or atomic clock. For radioactive isotopes, the rate of decay is approximated by a unit known as the half-life, whereby 50% of the parent isotope decays into daughter isotopes after a discrete time period. After another cycle of that time period, 50% of the remaining parent isotopes decays, and so on and so on. Carbon-14 decays spontaneously into Carbon-12, and has a half-life of 5,730 years, making it useful for age-dating archeological finds, artifacts, and fossils from the last Ice Age. Carbon is found in all forms of life on earth, and all organisms incorporate a small amount of Carbon-14 into their cells while living; after death, the amount of Carbon-14 is fixed and decays over time into Carbon-12. By measuring the relative amounts of Carbon-14 to Carbon-12, the age of a fossil or piece of wood may be determined. Beyond about 40,000 years, however, the amount of Carbon-14 is so small, that there is no longer a sufficient amount to make an accurate measurement. Among stable isotopes, the ratio of Oxygen-18 to Oxygen-16 is indicative of climate changes. Ancient air bubbles trapped in deeply buried glaciers may reveal ancient climates by comparing their respective oxygen isotope ratios to those of today.
The Secrets of Nuclear Power and Stars
Nuclear reactions are much more energetic than chemical reactions, because the forces unleashed by the center (nucleus) of the atom are much greater than those associated with the transfer of electrons between elements. There are two basic types of nuclear reactions, nuclear fission and nuclear fusion.
Nuclear fission is the process of splitting an atom, usually a large, heavy, unstable element such as Uranium. Controlled nuclear fission is the process used for what is known as "nuclear power." Nuclear fission creates heat, radiation, and radioactive by-products. The only useful part of nuclear fission is the heat, which is basically used to boil water to power steam turbines which generate electricity. The radiation and radioactive by-products are dangerous to humans and all life forms, and have presented a long-term environmental hazard ever since this process was discovered. Uncontrolled nuclear fission, involving a chain reaction causing the release of nuclear energy in an instant, is known as an atomic bomb.
Nuclear fusion is the process of the fusing together light elements into heavier elements; it is the process found in stars, including our sun.
On Earth, nuclear fusion has only been achieved on a large scale in an uncontrolled fashion, in devices known as hydrogen bombs. All hydrogen bombs have a fission (atomic bomb) trigger, because nuclear fusion requires a very high start-up temperature. Controlled nuclear fusion has only been achieved on a very small "bench top" scale because we do not currently have any technological means to create and contain the necessary high temperatures to start up and maintain nuclear fusion. We are able to reduce the start-up energy required for nuclear fusion by using deuterium and tritium mined from sea water. To contain the extremely high temperatures, no solid material is suitable; it requires a "bottle" made from a powerful magnetic field. Nuclear fusion currently requires more energy to start it up than we can get out of it, so it may not be practically achieved for many decades.
Cosmic Origins of the Elements
The Universe is estimated to contain 70% hydrogen
and 28% helium by mass. Of the 2% remaining, where did all
those other 100 or more known elements come from? The answer is that
these elements were created by exploding stars in a rare cataclysmic cosmic
event called a supernova. Stars the size of the Sun or smaller
may expand temporarily and then shrivel into a cool cinder called a dwarf
star at the end of their life spans. Stars that are at least 8 times
the mass of our Sun, however, eventually explode when they run out of hydrogen.
Stars normally maintain a balance between gravity (a force that pulls towards
the stars center) and an outward force created by its heat. At the
end of a star's life, when it loses most of its hydrogen, this balance
is upset and the star may become hotter as it rapidly fuses its helium
into heavier and heavier elements. After the elements fuse into iron,
the star explodes, and the explosion provides the energy and force to create
those elements heavier than iron. Because supernovas are relatively
rare events, elements heavier than iron are also rare. This also explains
why such heavy elements are also rare on the Earth.
Most elements do not exist in isolation from each other; they tend to form combinations. Combinations of two or more elements are called chemical compounds. A particle of a compound is called a molecule. There are certain elements that tend to form paired molecules, such as hydrogen, oxygen, nitrogen, chlorine, bromine, iodine, and fluorine. When elements are combined as compounds, the compound may sometimes be very different from the individual characteristics of its constituent elements. A good example is common table salt, NaCl (sodium chloride). Sodium is a poisonous metal that is so chemically active that it burns when mixed with water, and must be stored in kerosene. Chlorine is a poisonous gas so deadly that it was used as a chemical weapon during World War I and its military use was later banned by international treaty. Yet, the compound sodium chloride is safe enough to eat (in small amounts), and readily dissolves in water.
Ions are electrically charged atoms. Electrons in most elements orbit at different distances from their nuclei. The outermost electrons in some elements are prone to being dislodged, creating an electrical imbalance such that the resultant atoms become positively charged, or cations (pronounced "cat-eye-ons"). Other elements tend to "steal" electrons, causing a net negative electrical charge; such atoms are called anions (pronounced "ann-eye-ons").
Elements combine in discrete amounts, such the relative
proportions of each constituent elements may be represented by whole numbers.
The "glue" that holds elements together in compounds is electrical in nature,
and are called chemical bonds.
Some chemical compounds combine through the combination of ions
(called ionic bonding). Most other chemical compounds share
electrons (called covalent bonding).
There are certain elements that resist combining with other elements, called inert elements. Gold and platinum are considered inert, which is the reason why these metals do not tarnish, making them expensive and valuable. Other inert elements include the so-called noble gases: helium, neon, argon, krypton, and radon.
Earth's Distribution of Elements
Earth's distribution of elements is very different from that of the Sun, despite having a common origin. Furthermore, the earth's elements are not evenly distributed because the Earth is distinctively layered in different zones: the inner core, outer core, mantle, and crust. Most of the iron resides at the core of the Earth; the inner core is solid iron, while the outer core is composed of liquid iron. A complex interaction between the inner and outer core is believed to generate the Earth's magnetic field. Most of the volume of the Earth's interior is made up of the mantle, composed of silicate rocks high in iron and magnesium (known as peridotite). The outer shell of the Earth called the crust, is the only part of the Earth accessible for extracting natural resources. We are thus limited by the naturally occurring abundance of elements in the Earth's crust. This is a list of the 8 most abundant elements in the Earth's crust, from most common to least common:
Oxygen | Silicon | Aluminum | Iron | Calcium | Sodium | Potassium |
Magnesium
O Si Al Fe Ca Na K Mg
"O.C. Alfie Canackmeg"
An easy way to memorize the above list is to string
the chemical symbols together into a non-sensical name, "O.C. Alfie
Canackmeg."